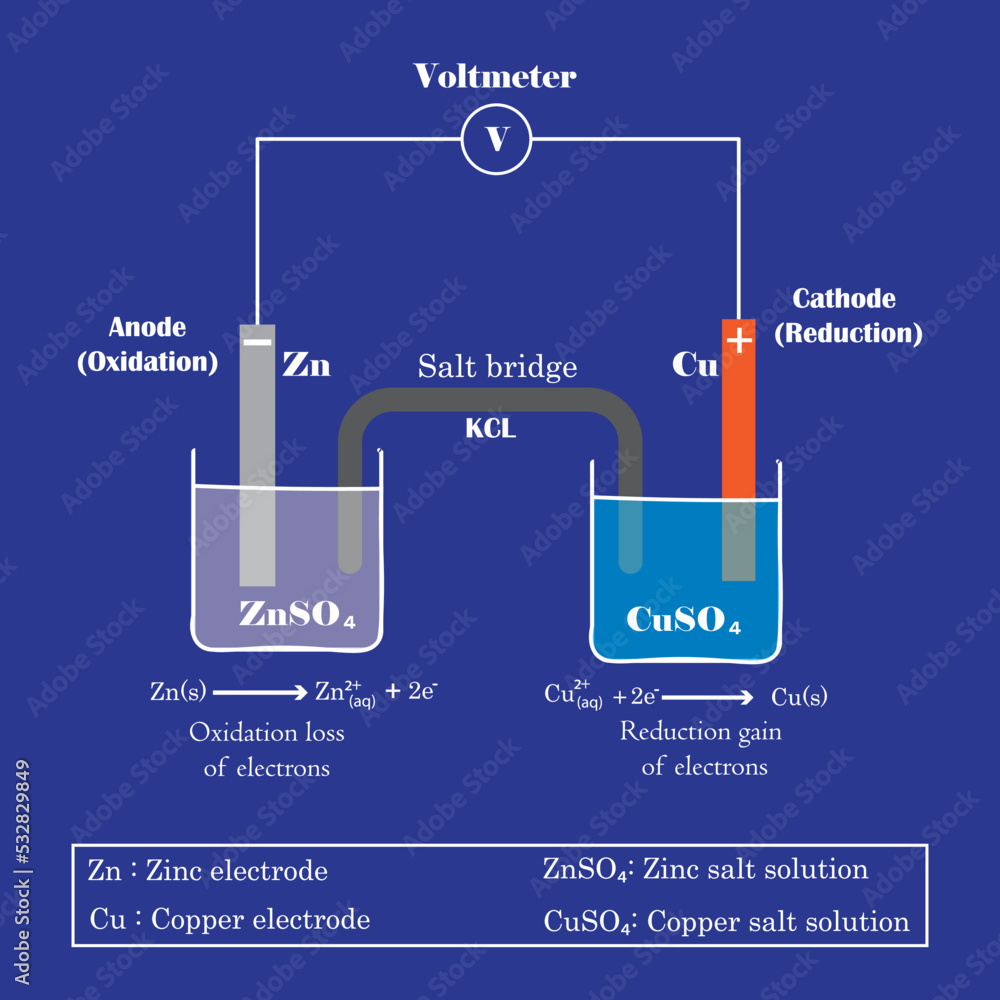
Electrochemical Cell Explain In Detail . an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. components of electrochemical cells. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. An electrochemical cell splits the oxidant and reductant in a manner that allows.
from stock.adobe.com
electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. This kind of cell includes the galvanic,. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. components of electrochemical cells.
Vecteur Stock Diagram of a electrochemical cell or Galvanic cell . The
Electrochemical Cell Explain In Detail electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. components of electrochemical cells. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. An electrochemical cell splits the oxidant and reductant in a manner that allows. This kind of cell includes the galvanic,. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Electrochemical Cell Explain In Detail an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. an electrochemical cell is a. Electrochemical Cell Explain In Detail.
From www.shutterstock.com
Diagrama de células electroquímicas. Célula galvánica vector de stock Electrochemical Cell Explain In Detail An electrochemical cell splits the oxidant and reductant in a manner that allows. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. This kind of cell includes the galvanic,. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. an apparatus that. Electrochemical Cell Explain In Detail.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemical Cell Explain In Detail electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. Web. Electrochemical Cell Explain In Detail.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Electrochemical Cell Explain In Detail components of electrochemical cells. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox. Electrochemical Cell Explain In Detail.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemical Cell Explain In Detail an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. components of electrochemical cells. This kind of cell includes the galvanic,. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. An electrochemical cell splits the oxidant and reductant in a. Electrochemical Cell Explain In Detail.
From slideplayer.com
Electrochemical Cells (aka Galvanic or Voltaic Cells) ppt download Electrochemical Cell Explain In Detail an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that. Electrochemical Cell Explain In Detail.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ Electrochemical Cell Explain In Detail components of electrochemical cells. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrochemical cell. Electrochemical Cell Explain In Detail.
From www.coursehero.com
[Solved] The diagram shows an electrochemical cell with a gold strip Electrochemical Cell Explain In Detail an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. components of electrochemical cells. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is a device that produces an electric current from energy. Electrochemical Cell Explain In Detail.
From 2012books.lardbucket.org
Describing Electrochemical Cells Electrochemical Cell Explain In Detail This kind of cell includes the galvanic,. components of electrochemical cells. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is called such because it utilizes the principles. Electrochemical Cell Explain In Detail.
From evrimagaci.org
Elektrokimyasal Piller Nasıl Çalışır? Galvanik (Voltaik) Hücrelerin Electrochemical Cell Explain In Detail an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. Web. Electrochemical Cell Explain In Detail.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrochemical Cell Explain In Detail electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. Web. Electrochemical Cell Explain In Detail.
From mavink.com
Diagram Of Electrochemical Cell Electrochemical Cell Explain In Detail This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical. Electrochemical Cell Explain In Detail.
From lessonschoolbathetic.z5.web.core.windows.net
Electrochemical Cells In Chemistry Electrochemical Cell Explain In Detail an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. components of electrochemical. Electrochemical Cell Explain In Detail.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemical Cell Explain In Detail an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. An electrochemical cell splits the oxidant. Electrochemical Cell Explain In Detail.
From www.researchgate.net
(A) Schematic representation of the electrochemical cell used for the Electrochemical Cell Explain In Detail components of electrochemical cells. an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional. Electrochemical Cell Explain In Detail.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Electrochemical Cell Explain In Detail This kind of cell includes the galvanic,. components of electrochemical cells. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. An electrochemical cell splits the oxidant and reductant in a. Electrochemical Cell Explain In Detail.
From www.thaigoodview.com
เซลล์ไฟฟ้าเคมี (Electrochemical cell) THAIGOODVIEW Electrochemical Cell Explain In Detail components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is called such because it utilizes the principles of electrochemistry and is the smallest functional unit of an electrochemical. an electrochemical cell is an apparatus or device that produces electric current from. Electrochemical Cell Explain In Detail.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge Electrochemical Cell Explain In Detail an electrochemical cell is an apparatus or device that produces electric current from chemical change and energy. This kind of cell includes the galvanic,. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. Electrochemical Cell Explain In Detail.